Triglycerides LR MONO
The characteristics of the “TRIGLYCERIDES LR MONO” kit are as follows:
Product Name: TRIGLYCERIDES LR MONO
Use: Kit for measuring triglycerides in serum or plasma.
Method: Enzymatic colorimetric GPO-PAP method.
Reference (REF): 10165
Batch (LOT): 5905
Contents: 2 x 100 ml (R1 2 x 100 ml, R3 1 x 8 ml)
Manufacturer and Address: SGM Italia srl - Via Olivadi 20 – 00126 Rome – Italy
Cholesterol LR MONO
The characteristics of the SGM Italia “CHOLESTEROL LR MONO Enzymatic Trinder method” total cholesterol enzymatic assay kit, based on similar information for cholesterol enzymatic assay kits, are as follows:
Assay method: Trinder enzymatic method (CHOD-POD, Phenol), using colorimetric spectrophotometry.
Reaction type: Endpoint.
Reading time: Approximately 5 minutes.
Kit composition: A single reagent and a standard for calibration. The kit contains 4 x 100 mL of reagent (R1) and 1 x 5 mL of standard.
Use: For in vitro diagnostic use only, reserved for professional laboratory use.
Stability/Storage: The serum is stable for several days between +2 and +8°C.
Precautions: Do not pipette by mouth; Follow precautions for handling laboratory reagents.
Sensitivity: The detection limit is approximately 1 mg/dL.
Interferences: Characteristics may vary depending on the analyzer used; interference with hemoglobin or bilirubin may be observed at certain concentrations.
GAMMA-GT LR
The GAMMA GT LR diagnostic kit is a kit for measuring gamma-GT in serum or plasma using the SZASZ method.
Main characteristics:
Product type: In vitro diagnostic (IVD) medical device.
Enzyme measured: Gamma-glutamyl transferase (Gamma-GT or GGT).
Method: SZASZ method.
Sample: Serum or plasma.
Product reference (REF): 10228.
Batch number (LOT): 5835D.
Contents: The kit includes two reagents: R1 (5 x 40 ml) and R2 (1 x 50 ml).
Storage conditions: Between 2°C and 8°C.
Manufacturer: SGM Italia srl, based in Rome, Italy.
Certification: CE marked.
Intended Use: For the diagnosis and monitoring of hepatobiliary diseases by healthcare professionals in the laboratory.
CK NAC LR
The CK NAC LR kit features are as follows:
Product Name: CK NAC LR.
Use: Kit for measuring creatine kinase (CK) in serum or plasma.
Method: Optimized kinetic method DGKC-IFCC.
Reference (REF): 10197.
Batch Number (LOT): 5908.
Contents: 60 + 15 ml, including R1 (1 x 60 ml) and R2 (1 x 15 ml).
Manufacturer/Distributor: SGM Italia srl, located in Via Olivadi 20 – 00126 Rome, Italy.
Clinical Significance: CK measurement is used in the diagnosis and treatment of myocardial infarction and muscle diseases such as progressive muscular dystrophy. N-acetylcysteine (NAC) acts as a CK activator.
Use: For in vitro diagnostic use only.
Albumin LR
The following are the characteristics of the ALBUMIN LR kit:
Product Name: ALBUMIN LR.
Use: Kit for the determination of albumin in serum or plasma.
Method: BCG (Bromocresol Green) colorimetric method.
Reference (REF): 1004054.
Contents:
R1: 1 x 250 ml.
STD (Standard): 1 x 8 ml.
Total Quantity (Cont.): 1 x 250 ml.
Batch Number (LOT): 5673B.
Use: IVD (In Vitro Diagnostic) – for in vitro diagnostic use.
Compliance: CE marked, indicating compliance with European standards.
Manufacturing: Made in Italy.
Manufacturer/Distributor: SGM Italia – Via Olivadi 20 – 00126 Rome – Italy.
LDH LR
The product “LDH LR Kinetic optimized method SCE” is a reagent for the determination of Lactate Dehydrogenase (LDH) with the following characteristics:
Method: Kinetic optimized method SCE. LDH catalyzes the reduction of pyruvate by NADH, and the rate of NADH reduction is measured photometrically, being proportional to the LDH concentration.
Reference (REF): 10118.
Batch number (LOT): 5965B.
Contents (Cont.): The kit contains 4 x 20 ml in total, consisting of:
R1: 4 x 16 ml.
R2: 1 x 16 ml.
Manufacturer: SGM Italia srl, located at Via Olivadi 20 – 00126 Rome – Italy.
Clinical significance: LDH is an enzyme present in the body, and elevated serum levels may indicate liver disease, myocardial infarction, kidney problems, muscular dystrophy, and anemia.
Glucose LR MONO
The following are the characteristics of the product “GLUCOSE LR MONO”:
Product Name: GLUCOSE LR MONO
Method: GOD-POD (Glucose Oxidase-Peroxidase) colorimetric enzymatic method
Use: IVD (In Vitro Diagnostics)
Certifications: CE Marked
Storage Conditions: Between 2°C and 8°C
Reference Number (REF): 10013
Batch Number (LOT): 5891
Contents: 4 x 100 ml (4 vials of 100 ml each)
Components: R1 4 x 100 ml (one reagent, also in 4 x 100 ml)
Manufacturer: SGM Italia srl - Via Olivadi 20 – 00126 Rome – Italy
RF With controls
The main product characteristics are as follows:
Product name: RF with controls (Latex agglutination test without predilution).
Reference (REF): 31013.
Contents: 100 tests.
Batch number (LOT): 6826.
Regulatory marking: IVD (In Vitro Diagnostic Medical Device) and CE.
Safety warning: May cause an allergic skin reaction (H317) and contains ProClin 950.
Manufacturer and address: SGM Italia – Via Olivadi 20 – 00126 Rome – Italy.
Hazard symbols: Two hazard pictograms are visible, one indicating a health hazard (human silhouette with a star) and the other a general hazard (exclamation mark).
Magnesium and Cholesterol
In vitro diagnostic reagents produced by SGM Italia. Their main characteristics are as follows:
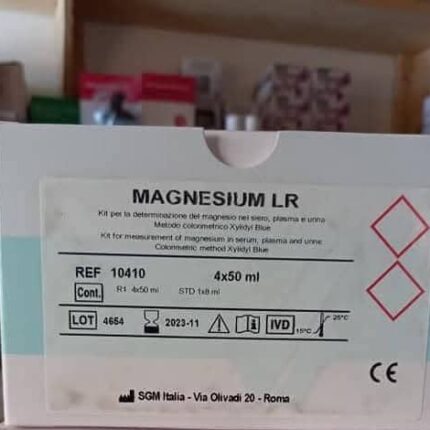
1. MAGNESIUM LR Kit
Intended use: Quantitative determination of magnesium in serum, plasma, and urine.
Method: Colorimetric, using Xylidyl Blue.
Reference (REF): 10410.
Contents: 4 x 50 ml (reagent R1) and one standard (STD).
Batch number (LOT): 4654.
Storage temperature: 15-25°C.
Certification: CE marked, indicating compliance with European standards.
Precautions: This is an in vitro diagnostic (IVD) medical device.
2. CHOLESTEROL LR MONO Kit
Intended use: Quantitative determination of cholesterol in serum or plasma.
Method: Enzymatic, using the Trinder method.
Reference (REF): 10023.
Contents: 4 x 100 ml (reagent R1) and one standard (STD).
Batch number (LOT): 4631.
Precautions: Indicates an in vitro diagnostic (IVD) medical device and safety warnings (hazard pictogram).
Certification: Marking
Disposable Cervical Brush
The sterile disposable cervical brush (or cytobrush) is primarily used for endocervical swabs during cervical cancer screening.
Its main features are:
Composition:
Shaft: Polystyrene or PVC with a stainless steel shaft.
Brush: Nylon bristles, soft to prevent damage to cervical tissue.
Dimensions (approximate):
Total Length: Approximately 18-19 cm.
Handle Length: Approximately 16 cm.
Brush Length: Approximately 20 mm (2 cm).
Brush Diameter: Approximately 5.5 mm to 8 mm.
Features:
Sterility: Sterile and single-use to prevent cross-contamination.
Design: Ergonomic brush head with soft synthetic bristles and a conical shape for optimal adaptation to the cervical opening.
Strength: Materials resistant to traction and handling.
Safety: Design to minimize discomfort and the risk of injury during collection.
Use:
Medical device designed for the collection of cervical cell samples for Pap smears.
Spinal Needle
Spinal needles have several important characteristics:
Needle Type: These are Quincke-type spinal needles used for spinal anesthesia or lumbar puncture.
Sterility and Single Use: They are sterile and designed for single use only to prevent infection.
Material and Design:
The needle barrel is made of thin-walled steel, facilitating the reflux of cerebrospinal fluid (CSF).
The needle hub is transparent to allow rapid visualization of CSF or blood reflux.
A notch or lug on the hub can indicate the bevel orientation.
Style:
The stylet fits snugly into the needle lumen to prevent coring and minimize tissue damage.
Its hub is colored differently depending on the needle’s outer diameter (standardized color coding).